| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 3, September 2015, pages 258-264
Prevalence of Maternal Genital Tract Colonization by Group B Streptococcus From Western Province, Taif, Saudi Arabia
Farzana Rizwan Araina, c, Nisreen Aref Al-Bezraha, Khadijah Y. Al-Aalib
aDepartment of Obstetrics & Gynecology, College of Medicine, Taif University, Saudi Arabia
bDepartment of Microbiology, College of Medicine, Taif University, Saudi Arabia
cCorresponding Author: Farzana Rizwan Arain, Department of Obstetrics & Gynecology, College of Medicine, Taif University, Saudi Arabia
Manuscript accepted for publication June 18, 2015
Short title: Maternal Genital Tract Colonization of GBS
doi: http://dx.doi.org/10.14740/jcgo341w
| Abstract | ▴Top |
Background: Group B streptococcus (GBS) or Streptococcus agalactiae are members of the normal flora of the female genital tract. Maternal colonization has been found to be a major risk factor for invasive GBS disease within 6 days of birth. GBS has become the major cause of bacterial infections in the perinatal period, including bacteremia, amnionitis, endometritis, and urinary tract infection in pregnant women as well as sepsis and meningitis in neonates and young infants. Infection of the new born may be acquired by the intra-amniotic route or directly during passage through the birth canal. This study was undertaken to determine the prevalence of GBS colonization in pregnant women attending the antenatal clinic of Al-Hada Armed Forces Hospital in Western province, Taif, Saudi Arabia. This paper was the first data on incidence of GBS in pregnant women at Western province, Taif, Saudi Arabia.
Methods: A total of 2,632 pregnant women were screened for GBS colonization between January and December 2014. Standard microbiological methods were used to isolate and identify GBS from vaginal and anorectal swabs obtained from study subjects. An antimicrobial susceptibility test was performed for all GBS isolates according to the criteria of the Clinical and Laboratory Standards Institute (CLSI) by disk diffusion method.
Results: A total of 632 out of 2,632 (24%) pregnant women were colonized by GBS. Statistically significant association was observed for GBS colonization with any of socio-demographic characteristics of the study subjects including age, occupation, number of antenatal clinic visits, and type of gravida. All GBS strains were susceptible to penicillin, ampicillin, vancomycin and gentamicin, erythromycin, tetracycline, ceftriaxone, chloramphenicol, ciprofloxacin, clindamycin, and norfloxacin. Resistance was observed in some strains against clindamycin (0.18%). The data from the present study showed that incidence of GBS in Saudi pregnant women increased rapidly. These results are the first record of the database in Saudi Arabia at western province with high prevalence of GBS in pregnant women.
Conclusion: This study showed that prevalence of GBS colonization was 24% among the study subjects. The finding of this study was comparable with findings reported from developed and developing countries. However, further epidemiological investigations should be done in different parts of the country (all provinces) in order to know the actual GBS colonization rate in pregnant women and to consider the use of intrapartum antibiotics prophylaxis for prevention of early onset GBS-neonatal diseases, considering the accelerated demand for reducing neonatal morbidity and mortality due to GBS.
Keywords: Genital tract; Group B streptococcus; Maternal; Colonization; Vaginal; Anorectal; Saudi Arabia; Al-Hada Armed Forces Hospital
| Introduction | ▴Top |
Group B streptococcus (GBS) or Streptococcus agalactiae is a Gram-positive bacterium that colonizes the human gastrointestinal and genital tracts. GBS is the most frequent bacterial pathogen in neonates [1]. Maternal colonization has been found to be a major risk factor for invasive GBS disease within 6 days of birth [2]. Approximately 10-40% of pregnant women are colonized with GBS in the vagina, the rectum, or both areas, and their main reservoirs are the genitourinary tract and the female alimentary tract [3].
GBS is a significant etiological factor of bacterial infections in neonates. Vertical transmission of GBS during labor or delivery may result in either invasive infection in the neonate during the first week of life or EOGBS disease [4]. The rate of peripartum transmission of GBS to newborns of colonized women is approximately 50%, and after that 1-2% of these newborns develop invasive GBS infection in the first week of life [5].
GBS, in the transition from commensal organisms to pathogens, will encounter diverse host environments and, thus, require coordinated control of the transcriptional responses to these changes. GBS may pose a threat for colonized women, most frequently those in the perinatal period, but above all, they can cause serious infections in fetuses and neonates. Babies are colonized with GBS mostly during deliveries or by ascending spread after rupture of membranes [6].
The risk of transmission of streptococci to a neonate can be as high as 70% and the frequency of disease contraction is 2 - 4 cases per 1,000 live births [7]. Infections in neonates are most frequently early-onset diseases (EODs) developing during the first 7 days of life and are characterized by high death rates. The most frequent clinical forms of neonatal GBS-caused infections are sepsis or pneumonia, and, less frequently, cerebrospinal meningitis [8].
In the 1990s in the USA, a high number of neonatal infections caused by GBS were noted, with early morbidity and a death rate up to 50% [1, 9]. This phenomenon was the reason why the Centers for Disease Control and Prevention (CDC) set guidelines in 1996 aimed at preventing GBS infections in neonates [10].
The American experience indicated that the most effective method to limit the number of infections in neonates is early prophylaxis based on a vaginal-rectal culture screening for GBS carriage of women during 35 - 37weeks of gestation, taking into consideration the existing infection risk factors [11].
In women colonized with GBS, the use of proper intrapartum antibiotic prophylaxis (IAP) was considered to be advisable, along with observation and possible diagnosis of their babies for GBS infection [12]. For IAP for women with GBS carriage, IV administration of penicillin G or, as an alternative, ampicillin, was recommended. In case of penicillin-allergic patients, in whom the use of cephalosporins was admissible, cephazolin should be administered.
In patients with a high risk of anaphylactic shock after the administration of penicillin, in the event of isolation of GBS strains with macrolide resistance phenotype, vancomycin should be administered, and in other cases erythromycin or clindamycin is preferred [13].
The aim of the study was to determine the prevalence of vaginal GBS (Streptococcus agalactiae) colonization in pregnant women from Western province, Taif, Saudi Arabia. In the Kingdom of Saudi Arabia, there is paucity of information regarding GBS carriage in pregnant women. To date, only few reports are in the literature. This paper was the first data on incidence of GBS in pregnant women at Western province, Taif, Saudi Arabia.
| Materials and Methods | ▴Top |
Study population
A total of 2,632 pregnant women attending the routine antenatal clinic of the Al-Hada Armed Forces Hospital were screened for GBS colonization. The samples were collected during 11 months, starting from January 2014 to December 2014. The approach was based on universal screening of all pregnant women for GBS colonization between 35 and 37 weeks of gestation [11, 14]. The age of the women is 15 - 44 years.
Sample collection
According to guidelines of the CDC [11] and the American College of Obstetricians and Gynecologists [12], swabs were taken from both the lower one-third of the vagina and the anal region using sterile cotton swabs by the attending midwife and placed in Amies transport medium (Oxoid, UK) and immediately transported to the Microbiology Laboratory of Al-Hada Armed Forces Hospital for culture. The vaginal and anorectal swabs were placed into 1 mL Todd-Hewitt broth (Saudi Prepared Media Laboratory (SPML), Riyadh, Saudi Arabia), supplemented with 10 μg/mL colistin and 15 μg/mL nalidixic acid (Biomerieux, France) to prevent growth of contaminants. The broth was incubated for 18 - 24 h at 35 - 37 °C and sub-cultured on 5% sheep blood agar (SPML, Riyadh, Saudi Arabia) and incubated overnight in 5% CO2 atmosphere for 18 - 24 h. All suspected GBS colonies (pin point, with narrow beta-hemolysis) were sub-cultured on blood agar (SPML, Riyadh, Saudi Arabia) and subjected for Gram stain and catalase test. All Gram-positive and catalase negative cocci isolates were tested for CAMP test and latex agglutination assay as a confirmatory testing for GBS (Oxoid, UK). Commercial identification kits were used to identify the isolates up to species level: API strep for identification of Streptococcus species (Analytab product, Plainview, NY), and Vitek system (bioMerieux, Inc., Durham, NC, USA); GPI card for identification of Streptococcus species.
Antimicrobial susceptibility testing
Susceptibility testing was performed to all GBS-positive samples. All procedures for disk susceptibility were performed according to the criteria of guidelines of Clinical and Laboratory Standards Institute (CLSI, 2013) [13]. Fresh subcultures of GBS were used after overnight growth on blood agar plate (SPML, Riyadh, Saudi Arabia). The inoculums were standardized by suspending colonies in sterile phosphate-buffered saline (pH 7.2) to achieve a turbidity of 0.5 McFarland standards. A sterile cotton swab was dipped into the bacterial suspension, elevated above the liquid and rotated several times against the inside wall of the tube to remove excess inoculums. Then the swab was inoculated on Mueller-Hinton agar plate (SPML, Riyadh, Saudi Arabia) supplemented with 5% de-fibrinated sheep blood to obtain confluent growth; antibiotic disks were placed and incubated at 35 °C with 5% CO2 atmosphere for 20 h.
Ten antibiotic disks (Oxoid) used were: penicillin G (P) (10 IU), erythromycin (E) (15 μg), tetracycline (TE) (30 μg), ampicillin (AMP) (10 μg), vancomycin (VA) (30 μg), ceftriaxone (CRO) (30 μg), chloramphenicol (C) (30 μg), ciprofloxacin (CIP) (5 μg), gentamicin (CN) (10 μg), norfloxacin (NOR) (10 μg), and clindamycin (2 μg).
The inducible clindamycin resistance (D-zone test) was tested with double disk diffusion tests. The diameters of inhibition zones were measured for each antibiotic and results were recorded as susceptible, intermediate, or resistant to the antimicrobial agents tested according to the criteria of guidelines of CLSI (2013) [13].
Quality control
To maintain the quality of data, every sample was processed in triplicates and every result was cross-checked by the principal investigator and the co-investigator. Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (ATCC 24923), and Streptococcus agalactiae (ATCC 13813) were used as quality control throughout the study for culture, Gram stain, and antimicrobial susceptibly testing. All the strains were obtained from the Remel Microbilogy products (Thermoscientific) [14].
Statistical analysis
The analysis was performed with Statistical Package for Social Sciences (SPSS) software version 15 (SPSS, Inc., Chicago, IL, USA). Prevalence figures were calculated for the total study population and separately by age groups. Occupation, number of antenatal clinic visit (ANC), and type of gravid were also recorded. Chi-square test was used to compare results between the pregnant women with different age groups. Statistical methods, descriptive statistics including statements of frequency with percentages, means ± standard deviations (SDs), and linear regression were analyzed. T-test, and differences were considered significant if P < 0.05 with confidence intervals (95% CI).
| Results | ▴Top |
Socio-demographic characteristics
The socio-demographic characteristics of 2,632 pregnant women screened for GBS colonization are presented in Table 1. The mean age of the participants was 25.6 years ranging 15 - 44 years. The majority of the participants were between the ages of 25 - 29 years (41.2%). Most of them were housewives (2,411, 91.6%) while the remaining were students (118, 4.5%), teachers (75, 2.8%), nurse (13, 0.5%), and doctor (6, 0.2%).
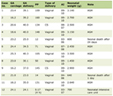 Click to view | Table 1. Socio-Demographic Characteristics of 2,632 Women Attending Obstetric Clinic Investigated for GBS, at AL-Hada Armed Forces Hospital in Taif, SA, 2014 |
Overall prevalence
Out of 2,632 pregnant women screened, 632 (24%) were found to be colonized with GBS.
Risk factors analysis
In the present data, a statistically significant association of GBS colonization was observed in connection with any of the socio-demographic characteristics of the study subjects by age groups: 15 - 19 years (P = 0.01), and 35 - 39 years (P = 0.05), as shown in Table 2.
 Click to view | Table 2. Variables Associated/Not Associated With Group B Streptococcus Colonization in Pregnant Women Attending Obstetric Clinic Investigated for GBS, at AL-Hada Armed Forces Hospital in Taif, SA, 2014 |
A statistically significant association of GBS colonization was observed in connection with any of the socio-demographic characteristics of the study subjects by occupation, where housewife gave high incidence (550, 22.8%) (P = 0.01) and doctors (5, 83.3%) (P = 0.005). In contrast, there is also statistically significant association of GBS colonization was observed in number of ANC visit (125, 41.7%) (P = 0.03).
Statistically no significant association of GBS colonization was observed in connection with any of the socio-demographic characteristics of the study subjects by type of gravida, neither primigravida nor multigravida.
The susceptibility pattern of 632 GBS isolated from pregnant women against 11 antimicrobial agents is presented in Table 3. All strains were susceptible to penicillin, ampicillin, vancomycin and gentamicin, erythromycin, tetracycline, ceftriaxone, chloramphenicol, ciprofloxacin, clindamycin, and norfloxacin. Resistance was observed in some strains against clindamycin (0.18%).
 Click to view | Table 3. Antimicrobial Susceptibility Pattern of 2,632 GBS Isolated From Pregnant Women Attending Obstetric Clinic Investigated for GBS, at Al-Hada Armed Forces Hospital in Taif, SA, 2014 |
| Discussion | ▴Top |
In the present study, the overall prevalence of GBS colonization among pregnant women was found to be 24%. Similar findings have been reported in other African countries such as in Malawi, Egypt, Zimbabwe, Gambia, and Tanzania; the prevalence of GBS in these countries ranges 16.5-23% [15-19]. However, low colonization rate was reported in a previous study conducted in Ethiopia (9%), some Latin American countries such as Peru (6%) and in Osijek, Croatia (24.6%) [20, 21].
Different studies conducted in Saudi Arabia showed results higher than present study, 70.4% [21], 31.6% [22] and 27.6% [23], whereas the incidence of GBS was uncommon in both mothers and infants at Abha Maternity Hospital, Abha, Kingdom of Saudi Arabia [24].
The data from the present survey showed that incidence of GBS in Saudi pregnant women increased rapidly. The rate of GBS colonization in this study is almost similar to the findings reported in some European countries. Two studies from Italy found a GBS colonization rate of 17.9% [25] and 18% [26]. Studies from Poland and Switzerland also found a colonization rate of 17.2% [27] and 21% [28], respectively.
Another study in a multicultural population of pregnant women in the Netherlands also showed a colonization rate of 21% [29]. However, lower GBS colonization rate has been reported in some Mediterranean countries such as Istanbul and Elazin in Turkey giving 8% [30] and 8.7% [31], respectively. A study conducted in a city of Northern Greece also found a low colonization rate of 6.6% [32], and Mozambique 1.8% [33].
The rate of GBS colonization in this study is almost similar to the findings reported in some European countries. The regional rates were Eastern Europe 19.7-29.3%, Western Europe 11-21%, Scandinavia 24.3-36%, and Southern Europe 6.5-32%[34], which is in agreement with the present study. The rates obtained in the present study, more or less, are similar with findings reported from most Asian countries like Thailand 16-18% [35, 36], Iran 20-26% [37, 38] and Saudi Arabia 27.6% [39], and 4.76% [24].
However, lower prevalence rates were reported in some Asian countries: in Hong Kong 10.4% [40] and Korea 3.9% [41], with similar findings from Australia 12.9% [42]. Knowledge about the risk factors contributing to GBS colonization in pregnant women is relevant to minimize the morbidity and mortality associated with maternal and neonatal GBS infections.
In the present study, a statistically significant association was observed for GBS colonization in the study subjects with any of the socio-demographic characteristics as outlined in Table 2. Similar findings have been reported in studies conducted elsewhere [43, 44]. However studies conducted in Athens and Hong Kong showed that GBS colonization rate was high among pregnant women who work outside either homes or those who had frequent visits of antenatal clinics [32, 40].
In the present study, the susceptibility pattern of 632 GBS isolated from pregnant women against 11 antimicrobial agents is presented in Table 3.
All strains were susceptible to penicillin, vancomycin, ciprofloxacin, and gentamicin (100%), clindamycin (99.8%), tetracycline, ceftriaxone, and norfloxacin (99.7%), ampicillin, erythromycin, and chloramphenicol (99.6%). However resistance was observed in some strains against clindamycin (0.18%). Similar findings have been reported in studies conducted in Tanzania [19], USA [45, 46], Canada [47] and Lebanon [19].
In the present study, the resistance against clindamycin was observed (0.18%); this study is almost similar to the findings reported in some countries. Clindamycin and erythromycin are recommended currently as second-line antimicrobial for intrapartum chemoprophylaxis for GBS infections for women who are allergic to penicillin [15]. Resistance of GBS to clindamycin and/or erythromycin has already been reported, ranging from 3% to 16% for erythromycin and from 2% to 15% for clindamycin [11, 18, 19].
The use of intrapartum antibiotics to prevent perinatal vertical transmission of GBS and early-onset neonatal sepsis has increased significantly after the CDC has published guidelines in 1996 which has recommended to screen the entire pregnant mothers before term (35 - 37 weeks of gestation) and to administer intrapartum prophylactic antibiotics to all of them. In addition, if the expectant mother’s status is not known at labor, chemoprophylaxis should be administered to all cases showing one or more of the main risk factors indicated by the CDC and to increase the efficiency of the existing guideline, the revised one was released in 2002, where it was stated that antibiotic of choice is either penicillin G or ampicillin [48].
Conclusion
The present study revealed a colonization rate of 24% with GBS among pregnant women attending the antenatal clinic of the Armed Forces Hospital, Taif, SA. Study results revealed a relatively high rate of GBS colonization in the population of pregnant women in Western province, Taif, Saudi Arabia. A statistically significant association was observed with any of the socio-demographic characteristics of the study subjects with GBS colonization by occupation, where housewife gave high incidence (550, 22.8%) (P = 0.01), and doctors (5, 83.3%) (P = 0.005) and number of ANC visit (125, 41.7%) (P = 0.03).
Statistically no significant association of GBS colonization was observed in connection with any of the socio-demographic characteristics of the study subjects by type of gravid, neither primigravida nor multigravida.
Large-scale epidemiological studies should be carried out in different parts of the country (all provinces in Saudi Arabia) in order to know the actual GBS colonization rate and GBS serotypes. Further assessment of risk factors associated with maternal GBS colonization is required.
Immunoprophylaxis and active immunization, in particular, are the most promising methods of preventing perinatal GBS disease in mothers and their infants, including late-onset disease.
Immunization of pregnant women with type III polysaccharide vaccines has resulted in adequate provision of functional antibody to the infants born to responders.
Considering the accelerated demand for reducing neonatal morbidity and mortality, due to GBS, it was surprising that so little research had been performed in this field in Saudi Arabia.
| References | ▴Top |
- Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817-826.
doi pubmed - Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, Mohle-Boetani J, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360(25):2626-2636.
doi pubmed - Picard FJ, Bergeron MG. Laboratory detection of group B Streptococcus for prevention of perinatal disease. Eur J Clin Microbiol Infect Dis. 2004;23(9):665-671.
doi pubmed - Beal S, Dancer S. Antenatal prevention of neonatal group B streptococcal infection. Rev. Gynaecol. Perinat. Pract. 2006;6:218-225.
doi - Kowalska B, Niemiec KT, Drejewicz H et al. Prevalence of group B streptococcal colonization in pregnant women and their newborns based on the results of examination of patients in the Obstetric and Gynecology Department of the National Research Institute of Mother and Child - a pilot study. Ginekol Pol. 2003;74:1223-1227.
pubmed - Wessels MR, Kasper DL: Group B Streptococcus. In: Gorbach SL, Bartlett JG, Blacklow NR (eds.), Infectious Diseases, 2nd ed. Philadelphia: WB Saunders Co.1998;1731-1735.
- Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, Harrison LH, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002;347(4):233-239.
doi pubmed - Kotarski J, Heczko PB, Lauterbach R, Niemiec T, Leszczynska-Gorzelak B, Polish Gynecological S. [Polish Gynecological Society's recommendations regarding diagnosis and prevention of streptococcus agalactiae infection in pregnant women and newborns]. Ginekol Pol. 2008;79(3):221-223.
pubmed - Johri AK, Paoletti LC, Glaser P, Dua M, Sharma PK, Grandi G, Rappuoli R. Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol. 2006;4(12):932-942.
doi pubmed - Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
pubmed - Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1-36.
pubmed - ACOG Committee Opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002;100(6):1405-1412.
doi - Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests- approved standard. 2006;M2-A9:Vol.26.No.1 2013.
- Musa Mohammed, Daniel Asrat, Yimtubezinash Woldeamanue, Demissie A. Prevalence of group B Streptococcus colonization among pregnant women attending antenatal clinic of Hawassa Health Center, Hawassa, Ethiopia2012. Ethiopian Journal of Health Development. 26(1).
- Dzowela T, Komolafe OO, Lgbigbia A. Prevalence of group B Streptococcus colonization in antenatal women at the Queen Elizabeth Central Hospital Blantyre - a preliminary study. Malawi Med J. 2005;17:97-99.
- Elbaradie SM, Mahmoud M, Farid M. Maternal and neonatal screening for Group B streptococci by SCP B gene based PCR: a preliminary study. Indian J Med Microbiol. 2009;27(1):17-21.
pubmed - Mavenyengwa RT, Afset JE, Schei B, Berg S, Caspersen T, Bergseng H, Moyo SR. Group B Streptococcus colonization during pregnancy and maternal-fetal transmission in Zimbabwe. Acta Obstet Gynecol Scand. 2010;89(2):250-255.
doi pubmed - Suara RO, Adegbola RA, Baker CJ, Secka O, Mulholland EK, Greenwood BM. Carriage of group B Streptococci in pregnant Gambian mothers and their infants. J Infect Dis. 1994;170(5):1316-1319.
doi pubmed - Joachim A, Matee MI, Massawe FA, Lyamuya EF. Maternal and neonatal colonisation of group B streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. 2009;9:437.
doi pubmed - Benchetrit LC, Fracalanzza SE, Peregrino H, Camelo AA, Sanches LA. Carriage of Streptococcus agalactiae in women and neonates and distribution of serological types: a study in Brazil. J Clin Microbiol. 1982;15(5):787-790.
pubmed - Muller-Vranjes A, Puntaric D, Curzik D, Sijanovic S, Topolovec Z, Kasac Z, Miskulin M. Prevalence and significance of vaginal group B streptococcus colonization in pregnant women from Osijek, Croatia. Coll Antropol. 2011;35(1):21-26.
pubmed - Nomura ML, Passini Junior R, Oliveira UM. Selective versus non-selective culture medium for group B streptococcus detection in pregnancies complicated by preterm labor or preterm-premature rupture of membranes. Braz J Infect Dis. 2006;10(4):247-250.
doi pubmed - Zusman AS, Baltimore RS, Fonseca SN. Prevalence of maternal group B streptococcal colonization and related risk factors in a Brazilian population. Braz J Infect Dis. 2006;10(4):242-246.
doi pubmed - Al-sunaidi M, Abong S, Al-sharani M, F.R.C.S., I.O. DAMOLE, FRCOG, and C.S.S. BELLO, Prevalence of Group B Streptococcus Colonization in Mothers and Babies at Abha General Hospital, Kingdom of Saudi Arabia. Med. J. Cairo Univ. 2011;79(2):163-165.
- Busetti M, D'Agaro P, Campello C. Group B streptococcus prevalence in pregnant women from North-Eastern Italy: advantages of a screening strategy based on direct plating plus broth enrichment. J Clin Pathol. 2007;60(10):1140-1143.
doi pubmed - Zamzami TY, Marzouki AM, Nasrat HA. Prevalence rate of group B streptococcal colonization among women in labor at King Abdul-Aziz University Hospital. Arch Gynecol Obstet. 2011;284(3):677-679.
doi pubmed - Strus M, Pawlik D, Brzychczy-Wloch M, Gosiewski T, Rytlewski K, Lauterbach R, Heczko PB. Group B streptococcus colonization of pregnant women and their children observed on obstetric and neonatal wards of the University Hospital in Krakow, Poland. J Med Microbiol. 2009;58(Pt 2):228-233.
doi pubmed - Rausch AV, Gross A, Droz S, Bodmer T, Surbek DV. Group B Streptococcus colonization in pregnancy: prevalence and prevention strategies of neonatal sepsis. J Perinat Med. 2009;37(2):124-129.
doi pubmed - Valkenburg-van den Berg AW, Sprij AJ, Oostvogel PM, Mutsaers JA, Renes WB, Rosendaal FR, Joep Dorr P. Prevalence of colonisation with group B Streptococci in pregnant women of a multi-ethnic population in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2006;124(2):178-183.
doi pubmed - Bararos I, Murat C, Mehmet V, Ismet T, Can K, Sukufe D, Ismail C, Yildiz P. The colonization incidence of group B streptococcus in pregnant women and their newborns in Istanbul. Pedatr Int. 2005;47:64-66.
doi pubmed - Ayata A, Guvenc H, Felek S, Aygun AD, Kocabay K, Bektas S. Maternal carriage and neonatal colonisation of group B streptococci in labour are uncommon in Turkey. Paediatr Perinat Epidemiol. 1994;8(2):188-192.
doi pubmed - Tsolia M, Psoma M, Gavrili S, Petrochilou V, Michalas S, Legakis N, Karpathios T. Group B streptococcus colonization of Greek pregnant women and neonates: prevalence, risk factors and serotypes. Clin Microbiol Infect. 2003;9(8):832-838.
doi pubmed - de Steenwinkel FD, Tak HV, Muller AE, Nouwen JL, Oostvogel PM, Mocumbi SM. Low carriage rate of group B streptococcus in pregnant women in Maputo, Mozambique. Trop Med Int Health. 2008;13(3):427-429.
doi pubmed - Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand. 2008;87(3):260-271.
doi pubmed - Tor-Udom S, Tor-Udom P, Hiriote W. The prevalence of streptococcus agalactiae (group B) colonization in pregnant women at Thammasat Hospital. J Med Assoc Thai. 2006;89(4):411-414.
pubmed - Kovavisarach E, Ying WS, Kanjanahareutai S. Risk factors related to group B streptococcal colonization in pregnant women in labor. J Med Assoc Thai. 2007;90(7):1287-1292.
pubmed - Fatemi F, Chamani-Tabriz L, Pakzad P, Zeraati H, Rabbani H, Asgari S. colonization rate of Group B Streptococcus (GBS) in Pregnant Women Using GBS Agar Medium. Acta Medica Iranica. 2008; 47:25-30.
- Rabiee S, Arab M, Mashouf YR. Epidemiologic pattern of vaginal colonization by Group B Streptococcus in pregnant women in hamadan, Central West of Iran. Iran J Med Sci. 2006;31:106-108.
- El-Kersh TA, Al-Nuaim LA, Kharfy TA, Al-Shammary FJ, Al-Saleh SS, Al-Zamel FA. Detection of genital colonization of group B streptococci during late pregnancy. Saudi Med J. 2002;23(1):56-61.
pubmed - Tsui HY, Ip M, Ng P, Sahota DS, Leung T, Lau T. Change in prevalence of group B Streptococcus maternal colonization in Hong Kong. Hong Kong Med J. 2009;15:C1-66.
- Uh Y, Kwon JY, Jang IH, Yoon KJ, Kim HG. Colonization rate of Group B Streptococcus in pregnant women and neonates. Korean J Clin Pathol. 1994;14:447-453.
- Garland SM, Kelly N, Ugoni AM. Is antenatal group B streptococcal carriage a predictor of adverse obstetric outcome? Infect Dis Obstet Gynecol. 2000;8(3-4):138-142.
doi pubmed - Collins TS, Calderon M, Gilman RH, Vivar A, Charache P. Group B streptococcal colonization in a developing country: its association with sexually transmitted disease and socioeconomic factors. Am J Trop Med Hyg. 1998;59(4):633-636.
pubmed - Costa AL, Lamy Filho F, Chein MB, Brito LM, Lamy ZC, Andrade KL. [Prevalence of colonization by group B Streptococcus in pregnant women from a public maternity of Northwest region of Brazil]. Rev Bras Ginecol Obstet. 2008;30(6):274-280.
doi pubmed - Manning SD, Foxman B, Pierson CL, Tallman P, Baker CJ, Pearlman MD. Correlates of antibiotic-resistant group B streptococcus isolated from pregnant women. Obstet Gynecol. 2003;101(1):74-79.
doi - Simoes JA, Aroutcheva AA, Heimler I, Faro S. Antibiotic resistance patterns of group B streptococcal clinical isolates. Infect Dis Obstet Gynecol. 2004;12(1):1-8.
doi pubmed - de Azavedo JC, McGavin M, Duncan C, Low DE, McGeer A. Prevalence and mechanisms of macrolide resistance in invasive and noninvasive group B streptococcus isolates from Ontario, Canada. Antimicrob Agents Chemother. 2001;45(12):3504-3508.
doi pubmed - Hannoun A, Shehab M, Khairallah MT, Sabra A, Abi-Rachid R, Bazi T, Yunis KA, Araj GF, Matar GM. Correlation between Group B Streptococcal genotypes, their antimicrobial resistance profiles, and virulence genes among pregnant women in Lebanon. Int J Microbiol. 2009;79:65-12.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







