| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 1, Number 1, February 2012, pages 24-27
A Case of Muscle Invasive Bladder Cancer Treated With Systemic Chemotherapy During Pregnancy
Stephen R Kennedya, c, Qurrat Mahmooda, Ananya Choudhurya, b, Jacqueline E Livseya
aDepartment of Clinical Oncology, Christie Hospital, Wilmslow Road, Withington, Manchester, UK M20 4BX, UK
bManchester Cancer Research Centre (MCRC), The University of Manchester, Wilmslow Road, Manchester, UK M20 4BX, UK
cCorresponding author: Stephen R Kennedy
Manuscript accepted for publication January 24, 2012
Short title: Muscle Invasive Bladder Cancer
doi: https://doi.org/10.4021/jcgo7e
| Abstract | ▴Top |
Bladder cancer during pregnancy is rare and there are no previous reports of the use combination chemotherapy being administered for muscle invasive bladder cancer (MIBC) during pregnancy. We report a case of muscle invasive bladder cancer diagnosed during pregnancy and treated during the second and third trimester with 2 cycles of systemic chemotherapy using Carboplatin and Adriamycin. We considered this novel combination regime to have activity against bladder cancer, but be safer in pregnancy than the standard regimes. After receiving chemotherapy, the neonate was delivered at 32 weeks by caesarean section and suffered no ill effects from the chemotherapy. The infant continues to develop normally. The patient went on to receive radical bladder radiotherapy.
Keywords: Pregnancy; Bladder cancer; Chemotherapy
| Introduction | ▴Top |
Muscle-invasive bladder cancer (MIBC) in pregnancy is rare. Between 1966 and 2005 there are 27 reports of bladder transitional cell carcinoma (TCC) in pregnancy in the published literature. Five of these were MIBC, but none received chemotherapy whilst pregnant [1, 2]. Standard radical treatment options for localised MIBC include radical cystectomy or radical bladder radiotherapy. Chemotherapy can be used in the neo-adjuvant, concurrent or adjuvant setting to improve outcomes. In pregnant patients with MIBC treatment options include termination of the pregnancy and proceeding as above, attempt a cystectomy whilst maintaining the pregnancy, systemic chemotherapy as a bridging treatment with definitive surgery or radiotherapy after delivery or no treatment whilst pregnant. Standard systemic treatments for bladder cancer have an unknown safety profile in pregnancy and we therefore chose a novel combination regime selected for known activity against bladder malignancy, but considered safer in pregnancy than standard regimes. Adriamcyin and carboplatin could be used again by clinicians treating a similar case.
| Case Report | ▴Top |
A 38 year old Caucasian female during her 2nd pregnancy (following IVF treatment) was referred for a routine abdominal ultrasound scan 18 weeks into her pregnancy. As well as confirming pregnancy this showed right sided hydronephrosis and 2 echogenic bladder wall masses. She had no history of bladder problems and denied haematuria. Her previous medical history included a longstanding myopathy (of unknown cause), severe kypho-scoliosis and respiratory muscle weakness requiring nocturnal ventilatory support. She had never smoked and had one child aged 6 years. Her mother had a similar myopathy and died aged 50 of bladder cancer.
Urine cytology was suspicious of malignancy. Flexible cystoscopy showed multiple bladder tumours, close to the urethra. She had a trans-urethral resection of her bladder tumour (TURBT) followed by a staging CT scan of thorax and MRI pelvis (Fig. 1, 2) (Sagittal and coronal view T2 MRI of pelvis showing MIBC and pregnancy). This confirmed a MIBC, histology grade 3 TCC (Fig. 3) (TURBT biopsy material confirming TCC). Overall staging was T3b N0 M0. The patient was counselled as to the risks of treatment for both herself and the foetus.
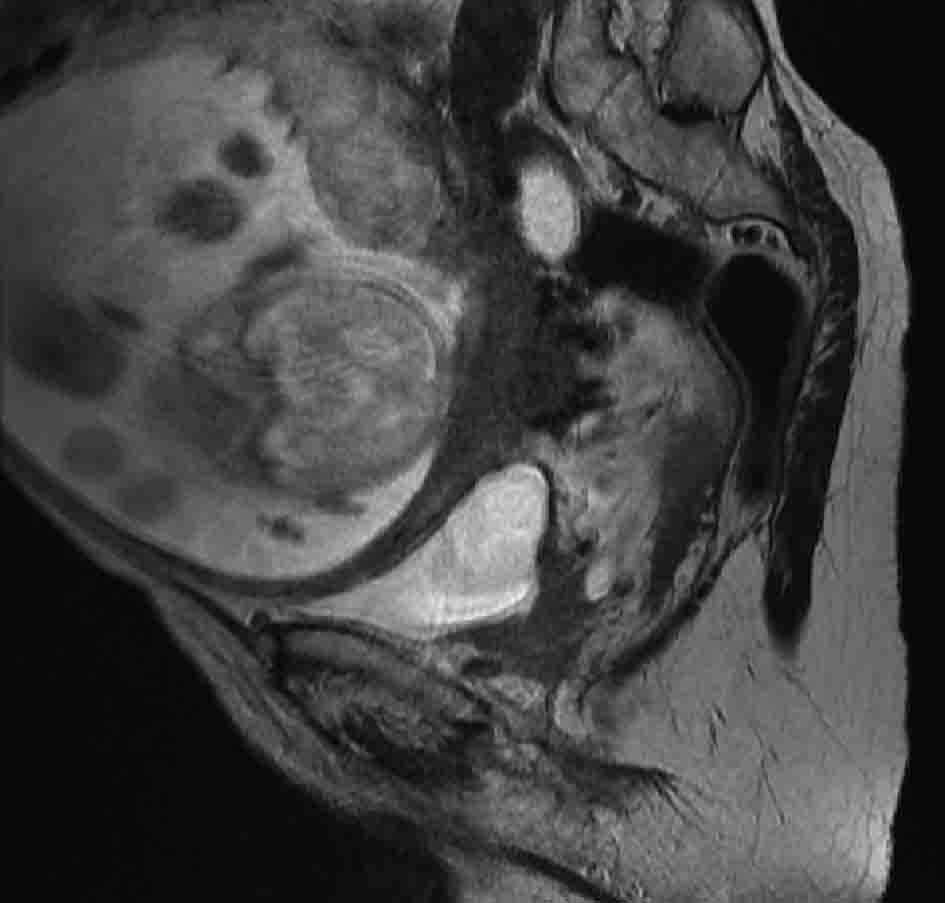 Click for large image | Figure 1. T2 weighted sagittal MRI image of pelvis, showing posterior bladder tumour and pregnancy. |
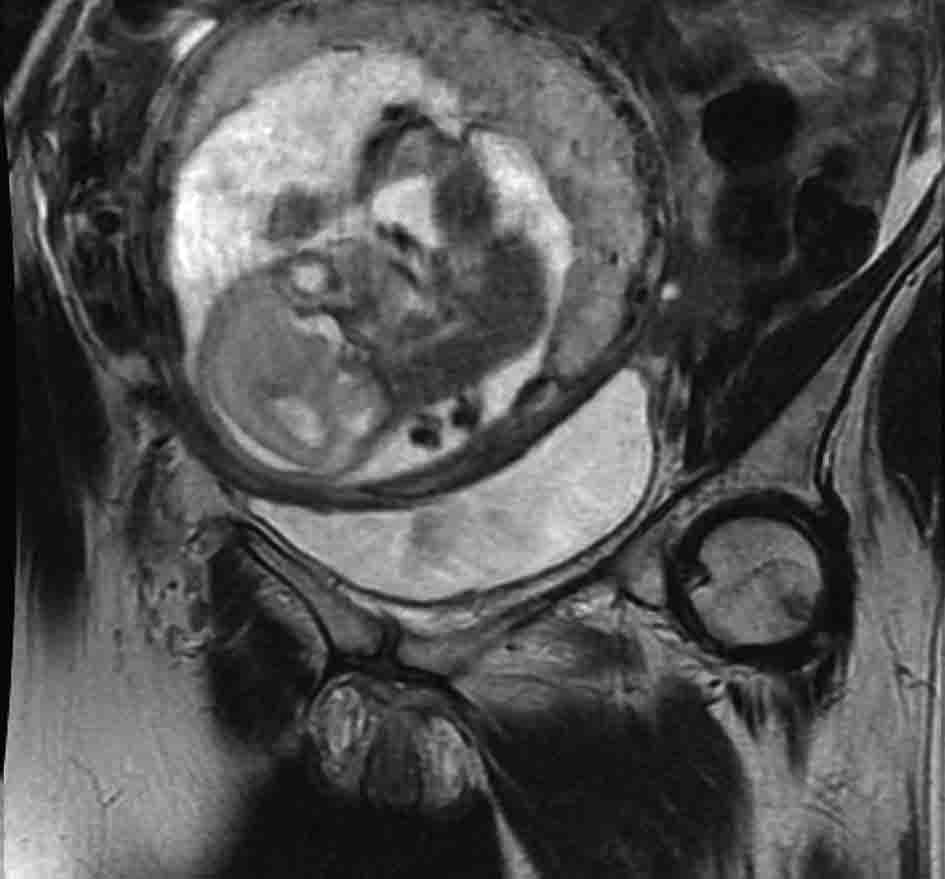 Click for large image | Figure 2. T2 weighted MRI pelvis, coronal showing MIBC and pregnancy. |
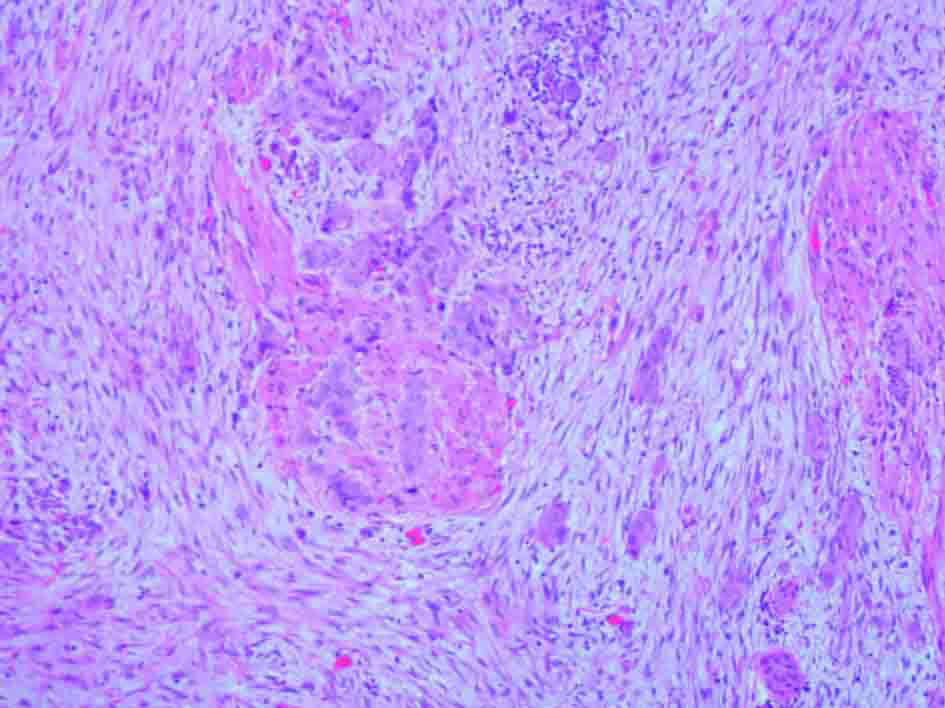 Click for large image | Figure 3. H&E stain of TURBT biopsy material confirming transitional cell carcinoma. |
When referred to the uro-oncology team she was 22 weeks pregnant, had significant but stable myopathy and remained asymptomatic. The following treatment options were considered (1) immediate cystectomy (plus or minus an abortion); (2) cystectomy after delivery at 28 weeks; (3) chemotherapy prior to delivery at 32 weeks followed by a cystectomy. The patient was keen to deliver as near to term as possible, but did not want to delay treatment of her bladder cancer. Since the predicted infant survival at 28 weeks is between 84 - 92 % [3], the patient opted to receive 2 three weekly cycles of chemotherapy prior to a planned elective caesarean section 3 weeks after her second chemotherapy (32 weeks). At 32 weeks infant survival is 97 - 100% [3].
The patient received adriamycin (50 mg/m2) and carboplatin (AUC 5) 3 weekly for 2 cycles. A lower segment Caesarean section (LSCS) 3 weeks post cycle 2 was planned followed by a cystectomy at the same time. After further discussion, it was decided that it would be safer to proceed to a further cycle of gemcitabine and cisplatin following LSCS and a delayed cystectomy.
Cycle 1 of adriamcyin and carboplatin began during the 26th week of pregnancy. Eleven days later the patient was found to be neutropenic (0.04 × 109/L), but asymptomatic. As there were no signs of infection, no antibiotics were given. The patient felt fatigued, but otherwise well. Her neutrophil count had recovered and she proceeded with cycle 2, three weeks after cycle 1, as planned. A 10% dose reduction was made due to the previous neutropenia. On day 5 of cycle 2, the patient was admitted to the intensive care unit with severe respiratory sepsis (WCC 8.4 ×109/L) and treated with intravenous antibiotics and non-invasive ventilation. On Day 26 of cycle 2 whilst still on intensive care, she had a LSCS (at 32 weeks) with an epidural. The neonate was born, 4lbs 10oz (2.09 Kg) with no complications.
Recovery post LSCS was slow and the patient was deemed unfit for an anaesthetic and cystectomy. Radical bladder radiotherapy was therefore considered. An MRI scan, four weeks post-partum showed a slightly larger bladder tumour, now invading the anterior vagina, without nodal disease. Six to 12 weeks post-partum, the patient had a pulmonary embolus and developed biventricular cardiac failure. With intensive medical management, the patient improved. A CT scan at 21 weeks post-partum showed a large atonic bladder, local extension of tumour into vagina and right sided hydronephrosis. A left sided necrotic obturator node was noted. At 23 weeks post-partum the patient received radical treatment using hypofractionated radiotherapy, 52.5Gy in 20 fractions over 4 weeks. A CT scan 7 weeks after radiotherapy showed a persistent mural thickening of the posterior bladder wall, left sided necrotic obturator node and right sided hydronephrosis. Further follow up revealed distant metastases and the patient unfortunately died 7 months after radiotherapy. The baby is alive and developing normally.
| Discussion | ▴Top |
Here we describe the use of a novel chemotherapy regime tailored to treat MIBC safely during pregnancy. The Advanced Bladder Cancer (ABC) Meta-analysis Collaboration 2005 showed that platinum based combinations are more effective in improving overall survival and disease free survival compared to single agent regimes [4]. The standard regimens for neo-adjuvant MIBC include: Gemcitabine 1000 mg/m2 days 1 and 8 plus Cisplatin 80 mg/m2 day 1 every 21 days [5] or accelerated MVAC (methotrexate 30 mg/m2 on day 1, vinblastine 3 mg/m2 on day 2, Adriamycin 30 mg/m2 on day 2, and cisplatin 70 mg/m2 on day 2, with G-CSF on days 4 - 11) [6]. The BA06 trial using neo-adjuvant cisplatin, methotrexate, and vinblastine (CMV) chemotherapy in patients with MIBC treated by cystectomy and/or radiotherapy, has shown a 6% improvement in 10 year survival [7].
Experience of using chemotherapy during pregnancy comes mainly from patients treated for breast and cervical cancer. There are a few reports of gemcitabine, a cytosine (pyrimidine) analogue being used in pregnancy, but given the crucial role of DNA synthesis in foetal development it was felt prudent to avoid it. Use of platinum agents in pregnancy have been reported more frequently and the side effect profile more predictable. Carboplatin an analogue of cisplatin has less toxicity in pregnancy [8].
Methotrexate, an anti-metabolite and Vinblastine a vinca-alkaloid have been used in pregnancy with documented toxicity to the fetus [9]. Doxorubicin (Adriamycin) is an anthracyline. Anthracylcines cause DNA breaks, which are poorly repaired during DNA replication and transcription. In the first trimester, the foetus is undergoing organogenesis, with rapid cell proliferation and DNA damage at this stage is more likely to lead to complications. Clinical case series of pregnant breast cancer patients confirm this pattern with anthracyclines, but show a safe profile when used in the 2nd and 3rd trimesters [10].
Considering the above, a combination of carboplatin and adriamcyin (doxorubicin) was thought to be safe and effective. Maternal neutropenia was the most significant side-effect and required a dose reduction. The episode of non-neutropenic respiratory sepsis could be attributable to chemotherapy, but advanced pregnancy, kyphoscoliosis and myopathy are likely to have contributed to her respiratory difficulties as well. Chemotherapy did not affect the birth or health of the neonate.
This is the first documented case of a pregnant patient being treated with combination chemotherapy for MIBC. The chemotherapy enabled a safe, controlled delivery of the infant with the highest chance of survival and normal development. It also allowed for the possibility for radical treatment to be delivered. Given trends towards increasing maternal age, bladder cancer during pregnancy may be seen more commonly. Adriamycin and Carboplatin could be used in a similar case in the future. This report demonstrates the complexity of treating a malignancy atypical in pregnant patients. We designed a rational systemic treatment based on first principles and by extrapolating evidence from other cases of malignancy presenting in pregnant patients e.g breast and cervical cancer.
| References | ▴Top |
- Spahn M, Bader P, Westermann D, Echtle D, Frohneberg D. Bladder carcinoma during pregnancy. Urol Int. 2005;74(2):153-159.
pubmed doi - Wax JR, Pinette MG, Blackstone J, Cartin A, McCrann DJ. Nonbilharzial bladder carcinoma complicating pregnancy: review of the literature. Obstet Gynecol Surv. 2002;57(4):236-244.
pubmed doi - Draper ES, Manktelow B, Field DJ, James D. Prediction of survival for preterm births by weight and gestational age: retrospective population based study. BMJ. 1999;319(7217):1093-1097.
pubmed doi - Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202-205; discussion 205-206.
pubmed doi - von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077.
pubmed - Sternberg CN, de Mulder PH, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, Witjes F, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19(10):2638-2646.
pubmed - Griffiths G, Hall R, Sylvester R, Raghavan D, Parmar MK. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-2177.
pubmed doi - Mir O, Berveiller P, Ropert S, Goffinet F, Goldwasser F. Use of platinum derivatives during pregnancy. Cancer. 2008;113(11):3069-3074.
pubmed doi - Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283-291.
pubmed doi - Germann N, Goffinet F, Goldwasser F. Anthracyclines during pregnancy: embryo-fetal outcome in 160 patients. Ann Oncol. 2004;15(1):146-150.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







